Trypanosoma cruzi
Trypanosoma cruzi is a hemoflagellate protozoan parasite primarily found in the southern USA and central and south America.
Summary
Trypanosoma cruzi - Chagas disease
Trypanosoma cruzi is a hemoflagellate protozoan parasite primarily found in the southern USA and central and south America. Serosurvey in foxhounds in the northeast of North America and shelter dogs in Texas has demonstrated widespread exposure to Trypanosoma cruzi - the cause of Chagas disease (also known as American trypanosomiasis) in people and animals, including dogs. In this disease, which is often fatal, the parasite attacks smooth and cardiac muscle. Trypanosoma cruzi has an indirect life cycle requiring triatomine bugs as intermediate hosts. The bugs become infected during blood-feeding. The parasites develop in the gut of the bug and leave in the feces. Congenital infection with T. cruzi also occurs, as do transmission by ingestion of tissues from infected animals, by blood transfusion and organ transplantation (both significant public health concerns in North America), and by the ingestion of food contaminated with parasites from the faeces of infected bugs. Locally acquired infections with T. cruzi and cases of Chagas disease are very rare in people in the United States. They do occur in domestic dogs, however, and serosurveys indicate that exposure is common among a variety of free-ranging mammals. Diagnosis in dogs is difficult, as early infections are generally not detected, and relies on clinical signs (cardiomegaly, megesophagus), serology, and possibly PCR on whole blood. Treatment is also difficult, generally recommended only in acute infections, with drugs that are toxic.
Taxonomy
Phylum: Euglenozoa
Class: Kinetoplastea
Order: Trypanosomatida
Family: Trypanosomatide
Trypanosomes are in the same class as Leishmania, and both are hemoflagellates. T. cruzi is a stercorarian trypanosome, which, unusually for this group, is pathogenic. Other stercorarians are nonpathogenic in cattle (T. theileri, transmitted by tabanid flies and ticks), sheep (T. melophagium, transmitted by keds), and cervids (T. cervi, transmitted by tabanid flies). These are distinct from the salivarian trypanosomes, which cause nagana in cattle and sleeping sickness in people in Africa (transmitted by tsetse flies), surra in many animals in Africa, Asia, and Latin America, and dourine (T. equiperdum, sexually transmitted) which has been eradicated in horses in North America and Europe.
Note: Our understanding of the taxonomy of parasites is constantly evolving. The taxonomy described in wcvmlearnaboutparasites is based on Deplazes et al. eds. Parasitology in Veterinary Medicine, Wageningen Academic Publishers, 2016.
Morphology
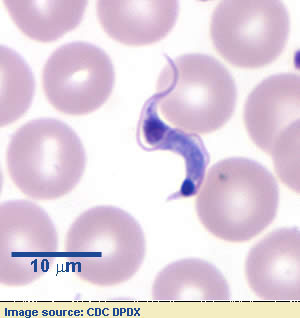
Host Range and Geographic Distribution
Chagas disease naturally occurs between 40°N (southern United States) and 45°S latitude (near the bottom of South America) and below 1500m elevation. The triatomine bugs are the sole vectors of T. cruzi and the distribution of Chagas disease largely mirrors the geographic distribution of the triatomine bugs. The most important hosts of Chagas disease are humans, dogs, opossums and to an extent armadillos. Humans and dogs are distributed throughout the Americas. Armadillos and Opossums also have a significant geographic range in the America’s. Many other animals have been found to be infected with T. cruzi, as many as 180 small wild animals to date.
Life Cycle
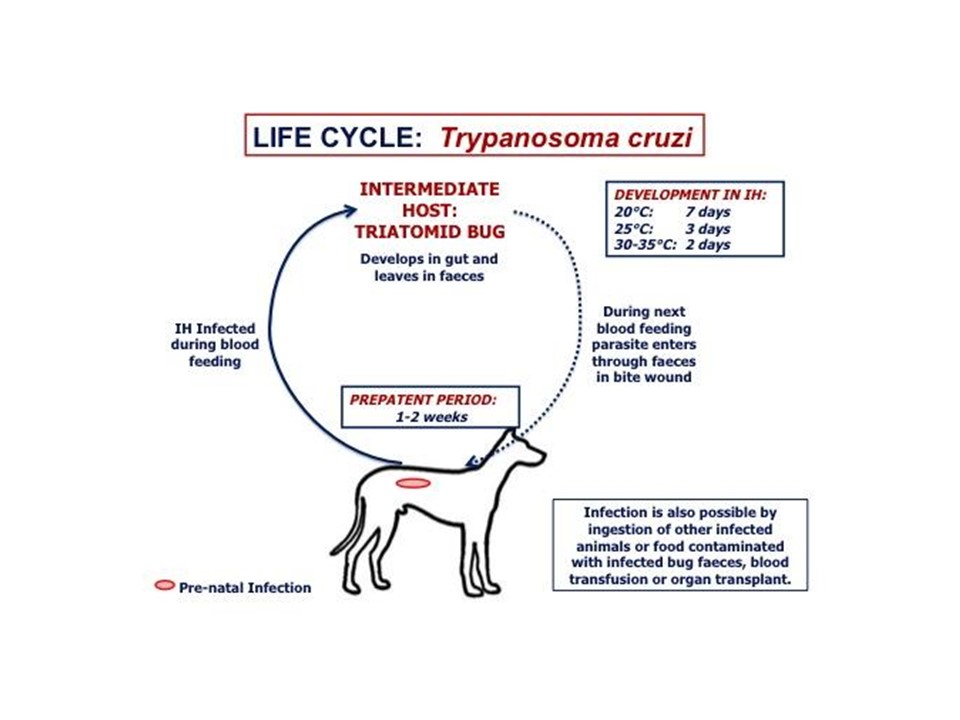
The triatomine bug takes a blood meal from an infected host, ingesting circulating trypomastigotes, which are infectious to the triatomine bug. The harsh environment of the bug's stomach will kill a significant portion of the ingested trypanosomes. The remaining trypomastigotes will differentiate into amastigotes, which lengthen into mid-log epimastigotes. The epimastigotes will continue to lengthen into late-log epimastigotes as the blood meal is depleted. The epimastigotes will replicate via binary fission in the midgut of the bug. The epimastigotes will attach to the cuticle lining in the rectum and go through metacyclogenesis and transform into non-proliferative metacyclic trypomastigotes.
The infected triatomine bug defecates on the victim shortly after feeding. The trypomastigotes from those feces can easily be scratched into the bite wound, rubbed into a mucous membrane or ingested as the animal cleans itself. The parasite may also enter the animal by eating an infected triatomine bug, blood transfusions, and vertical transmission (mother-to-offspring). Once the trypomastigotes have entered the host they will either remain in circulation to disseminate throughout the body or enter a host cell. The trypomastigotes invade a wide variety of phagocytic and non-phagocytic nucleated cells where they differentiate into amastigotes, which then replicate via binary fission. Amastigotes in the cell cytoplasm elongate, reacquire their flagella and develop into trypomastigotes. The trypomastigotes will then begin to invade adjacent cells or enter the blood and lymphatic system and differentiate extracellularly. Trypomastigotes do not replicate and must differentiate into the amastigote stage to replicate within the host.
Epidemiology
There is a vast number of vertebrates that can be infected with T.cruzi (~180) and the domestic, peridomestic, and sylvatic populations of triatomine bugs allow for continual transmission. Recent estimates show that around 60% of triatomine bugs in Texas are infected with T. cruzi. Another study found the infection rate in triatomine bugs in 17 US states ranges from 20-70% with an average of 54%. A recent prevalence study conducted on 540 dogs from 20 animal shelters in southern Louisiana found a 6.9% seropositivity and a 15.7% PCR positivity, which was unexpectedly high. This emphasizes the importance of taking a detailed travel history when treating dogs with electrocardiographic abnormalities or cardiomegaly. There are no triatomine bugs in Canada, so there is no autochthonous transmission, but people and pets may bring the parasite from endemic areas to Canada.
Diagnosis
Index of suspicion should be high in a dog with compatible clinical signs and a travel history including Texas and other regions of the southern USA, central and southern America. In people, different diagnostic methods are required depending on the stage of infection. In the acute stage (prior to seroconversion) parasitological examination (microscopy) is the most common method, with molecular methods being second (PCR). Xenodiagnosis and hemoculture used to be common methods but due to low sensitivity and specificity, they are uncommon today. Direct parasitological examination is done by looking for trypomastigotes in peripheral blood and is preferentially done using blood films or buffy coat smears (wet mounts, preferably stained). Direct examination is unreliable because parasitemia may be too low for detection. PCR on whole blood is the best method, but requires trained personnel and a well-equipped laboratory, and is generally only positive in acute infection.
Once the infected individual has progressed to the indeterminate or chronic phase (which is almost always when a dog would present for care), the best diagnostic methods are serological. Antibodies are typically detectable in animals three weeks after infection; the anti-T.cruzi antibodies will remain in the animal for life. At least two serological assays are required for definitive diagnosis and if there is discordance between the two tests, a third confirmatory test must be used. The four most common serological assays used are Enzyme linked immunosorbent assays (ELISAs), Indirect immunofluorescence assay (IFA), Indirect hemagglutination assay (IHA), and an Immunochromatographic qualitative assay (rapid test) which is commercially available and has been used in dogs.Treatment and Control
Treatment options in humans and dogs are equally poor. The two most commonly used pharmaceuticals are Benznidazole and Nifurtimox. Benznidazole is considered to be safer and more efficacious in humans and dogs than Nifurtimox. Both of these Nitroheterocyclic compounds come with a serious risk of adverse events, including neurotoxicity and carcinogenicity. Initiating treatment with either of these drugs must consider the pros and the cons. The standard dosing regimen with Benznidazole is 5-10mg/kg by mouth every 24 hours for 2 months, but is not commonly used in dogs. Vomiting is the main observable adverse event in dogs being treated with Benznidazole. Other “Azoles” have been tested in the treatment of humans and animals with varying success rates. No other drugs to date have shown to be more efficacious than Benznidazole.
Treatment of Chagas disease should ideally be initiated in the acute stage of the disease. If treatment is not started in the acute stage, curative rates drop significantly, below 50% in most cases. This poses a problem because most of the time the acute stage is silent and the individual has little or no symptoms. Detection of Chagas disease is typically in the chronic stage when the individual is exhibiting symptoms (cardiomegaly, megaesophagus, megacolon). Treatment with Nitroheterocyclic compounds can often do more harm than good in this stage, thus symptomatic treatment is more appropriate in most cases.
Control measures for prevention of T. cruzi infection in endemic regions are less than ideal. Spraying of homes that are infested can be extremely effective if the local vector only has a domestic cycle (e.g., Rhodnius prolixus), but if the vector has a peridomestic cycle (e.g., Triatoma dimidiata) the spraying will work temporarily and the vector will reinfect the home. There is no vaccine currently available for the prevention of Chagas disease. Housing dogs inside at night, away from triatomine bugs, can help reduce the risk of the animal becoming infected.
Publice Health Significance
In terms of DALYs (disability adjusted life years) Chagas disease is the most important parasitic disease in people in Latin America. 65-120 million people live in at-risk areas and 6-12 million are presumed to be infected. Annual deaths are estimated to be between 10,000-50,000. With no currently available vaccines and a low efficacy in treating the disease beyond the acute stage, Chagas disease poses a significant issue in humans and dogs, the only mammals that show signs of disease. Dogs that roam outdoors and sleep inside the home at night have the potential to bring triatomine bugs into the house, which can infect the human owners. Another significant barrier to effective treatment is when infected humans and dogs migrate from an endemic area to a non-endemic area because the doctors and veterinarians in their new locales often will not consider Chagas disease or even know the disease well enough to identify it. Assessing the true burden of Chagas disease is difficult because many infections are asymptomatic and the hyperendemic areas are also the poorest, which lack sufficient healthcare infrastructure. For these reasons, 99% of human Chagas disease cases are estimated to be undiagnosed and one can reasonably assume that almost 100% of Chagas disease cases in dogs would be undiagnosed in these areas.
References
Yoshida, Nobuko, Kevin M. Tyler, and Martin S. Llewellyn. "Invasion Mechanisms among Emerging Food-borne Protozoan Parasites." Trends in Parasitology 27, no. 10 (2011): 459-66. doi:10.1016/j.pt.2011.06.006.
Barr, Stephen C. "Canine Chagas Disease (American Trypanosomiasis) in North America." Veterinary Clinics of North America: Small Animal Practice 39, no. 6 (2009): 1055-064. doi:10.1016/j.cvsm.2009.06.004.
Kirchhoff, Louis V., and Richard D. Pearson. "The Emergence of Chagas Disease in the United States and Canada." Current Infectious Disease Reports 9, no. 5 (2007): 347-50. doi:10.1007/s11908-007-0053-9.
Nunes MCP, Beaton A, Acquatella H, Bern C, Bolger AF, Echeverría LE, et al. Chagas Cardiomyopathy: An Update of Current Clinical Knowledge and Management: A Scientific Statement From the American Heart Association. 2018;138:e169-e209. doi: 10.1161/CIR.0000000000000599.
PAHO, WHO. Chagas Disease in the Americas: A Review of the Current Public Health Situation and a Vision for the Future. Conclusions and Recommendations. 2018.
Triana DRR, Mertens F, Zúniga CV, Mendoza Y, Nakano EY, Monroy MC. The Role of Gender in Chagas Disease Prevention and Control in Honduras: An Analysis of Communication and Collaboration Networks. EcoHealth. 2016;13(3):535- 48. doi: 10.1007/s10393-016-1141-9.
WHO. More information on Chagas disease transmission. World Health Organization; 2016. Available from:https://www.who.int/chagas/disease/home_transmission_more/en/
Patterson JS, Guhl F. Geographical Distribution of Chagas Disease. 2010. 83- 114 p.
Balouz V, Agüero F, Buscaglia CA. Chagas disease diagnostic applications: present knowledge and future steps. Advances in Parasitology. 2017;97(1). doi: 10.1016/bs.apar.2016.10.001.
Onyekwelu CK. Life Cycle of Trypanosoma cruzi in the Invertebrate and the Vertebrate Hosts. IntechOpen; 2019.
Melo RdFP, Guarneri AA, Silber AM. The Influence of Environmental Cues on the Development of Trypanosoma cruzi in Triatominae Vector. Frontiers in Cellular and Infection Microbiology. 2020;10:27-. doi: 10.3389/fcimb.2020.00027.
Tyler KM, Olson CL, Engman DM. The life cycle of Trypanosoma cruzi. 7 ed: Kluwer Academic Publishers (Springer); 2003. p. 1-11.
Centers for Disease. American trypanosomiasis 2019. Available from:https://www.cdc.gov/dpdx/trypanosomiasisamerican/index.html.
Salgado ARU, Sabillon N. Miocardiopatía aguda por Chagas: Reporte de Caso y Revisión de la Literatura. Revista de Ciencias Forenses de Honduras. 2017;3(1):19-24.
Francisco AF, Jayawardhana S, Lewis MD, Taylor MC, Kelly JM. Biological factors that impinge on Chagas disease drug development. Parasitology. 2017;144:1871-80. doi: 10.1017/S0031182017001469.
Parra-Henao G, Cardona ÁS, Quirós-Gómez O, Angulo V, Alexander N. House-level risk factors for Triatoma dimidiata infestation in Colombia. American Journal of Tropical Medicine and Hygiene. 2015;92(1):193-200. doi: 10.4269/ajtmh.14-0273.
Ponce C, Zeledón R. La endermedad de Chagas en Honduras. Boletín de la Oficina Sanitaria Panamericana (OSP). 1973;75(3).
Meymandi S, Hernandez S, Park S, Sanchez DR, Forsyth C. Treatment of Chagas Disease in the United States. Current Treatment Options in Infectious Diseases. 2018;10(3):373-88. doi: 10.1007/s40506-018-0170-z.
Centers for Disease Control and Prevention. "CDC - DPDx - American Trypanosomiasis." Centers for Disease Control and Prevention. 2021. https://www.cdc.gov/dpdx/trypanosomiasisamerican/index.html.
Zecca, Italo B., Carolyn L. Hodo, Sarah Slack, Lisa Auckland, and Sarah A. Hamer. "Trypanosoma Cruzi Infections and Associated Pathology in Urban-dwelling Virginia Opossums (Didelphis Virginiana)." International Journal for Parasitology: Parasites and Wildlife 11 (2020): 287-93. doi:10.1016/j.ijppaw.2020.03.004.
Santos, Filipe Martins, Wanessa Texeira Gomes Barreto, Gabriel Carvalho De Macedo, Juliana Helena Da Silva Barros, Samanta Cristina Das Chagas Xavier, Carolina Martins Garcia, Guilherme Mourão, Jader De Oliveira, Aline Ribeiro Rimoldi, Grasiela Edith De Oliveira Porfírio, Gisele Braziliano De Andrade, Lívia Perles, Marcos Rogério André, Ana Maria Jansen, and Heitor Miraglia Herrera. "The Reservoir System for Trypanosoma (Kinetoplastida, Trypanosomatidae) Species in Large Neotropical Wetland." Acta Tropica 199 (2019): 105098. doi:10.1016/j.actatropica.2019.105098.
Dogs, Chagas Disease in, Catherine Barnette, and Contributors: Catherine Barnette. "Chagas Disease in Dogs." Vca_canada_corporate. n.d. https://vcacanada.com/know-your-pet/chagas-disease-in-dogs.
Saunders, Ashley B., and Sarah A. Hamer. "Chagas Disease in Dogs: Transmission, Diagnosis, Treatment, and Prevention." Today's Veterinary Practice. June 26, 2020. Accessed September 11, 2021. https://todaysveterinarypractice.com/chagas-disease-dogs/.
Companion Animal Parasite Council. "Trypanosomiasis." Companion Animal Parasite Council. 2013. https://capcvet.org/guidelines/trypanosomiasis/.
Cunha, Eleonora Lima Alves, Fernanda Karoline Vieira Da Silva Torchelsen, Lucas Maciel Cunha, Maykon Tavares De Oliveira, Kátia Da Silva Fonseca, Paula Melo Abreu Vieira, Cláudia Martins Carneiro, and Marta De Lana. "Benznidazole, Itraconazole and Their Combination in the Treatment of Acute Experimental Chagas Disease in Dogs." Experimental Parasitology 204 (2019): 2544-552. doi:10.1016/j.exppara.2019.05.005.
The Center for Food Security & Public Health. "Chagas Disease." Iowa State University. 2013. https://www.cfsph.iastate.edu/FastFacts/pdfs/chagas_F.pdf.
U.S. Food and Drug Administration. Highlights of Prescribing Information - Benznidazole. FDA, 2017.
Elmayan, Ardem, Weihong Tu, Brandy Duhon, Preston Marx, Wendy Wolfson, Gary Balsamo, Claudia Herrera, and Eric Dumonteil. "High Prevalence of Trypanosoma Cruzi Infection in Shelter Dogs from Southern Louisiana, USA." Parasites & Vectors 12, no. 1 (2019). doi:10.1186/s13071-019-3572-y.
Hamer, Sarah. "Chagas Disease in Dogs (2018)." Texas A&M University - Veterinary Medicine & Biomedical Sciences - CVMBS News. 2018. https://vetmed.tamu.edu/news/pet-talk/chagas-disease-in-dogs-2018/.
References
Barr SC (2009) Canine Chagas disease (American trypanosomiasis) in North America. Veterinary Clinics of North America Small Animal Practice 39: 1055-1064.
Kirchhoff LV et al. (2007) The emergence of Chagas disease in the United States and Canada. Current Infectious Disease Reports 9: 347-350.

