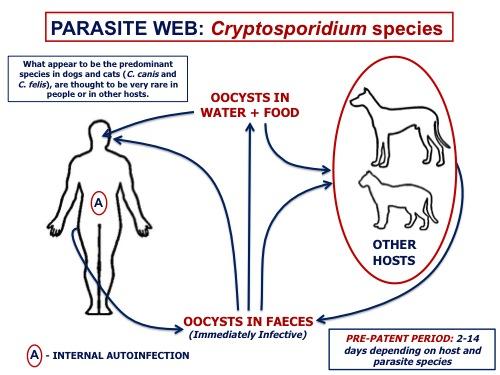
Cryptosporidium species
Cryptosporidium spp. are gastrointestinal coccidians (although the taxonomy is controversial) of a wide range of vertebrates (mammals, birds, amphibians, reptiles, and fish). Surveys from several locations around the world based on fecal examination for Cryptosporidium oocysts have demonstrated a prevalence of up to 40 per cent in dogs and cats.
Summary
Cryptosporidium spp. are gastrointestinal coccidians (although the taxonomy is controversial) of a wide range of vertebrates (mammals, birds, amphibians, reptiles, and fish). Some species are host specific (like C. canis in dogs and C. felis in cats), while others are generalists and may transmit between people and animals (like C. parvum). The life cycle of Cryptosporidium involves asexual (merogony) and then sexual (gametogony) reproduction within the microvilli on the luminal surface of enterocytes of the small intesttine, resulting in the production of thick- and thin-walled sporulated oocysts that are immediately infective. Autoinfection may occur, especially in immunocompromised hosts. Infection of dogs and cats with Cryptosporidium is much more common than is disease. Most cases are in young animals, with clinical signs usually diarrhea, dehydration and loss of weight. Diagnosis is best accomplished by a diagnostic lab using special stains, immunofluorescent assays, and/or PCR, as visualization of the very small oocysts is very difficult on routine fecal flotation.
There are no drugs approved in Canada approved for Cryptosporidium spp. infection in dogs or cats, which are largely self-limiting in immunocompetent hosts. Extralabel paromomycin, tylosin and azithromycin have been tried with some success in cats, but there are concerns about the safety of paromomycin.
The Cryptosporidium species infecting dogs and cats have been isolated from people only rarely, and most human infections have been in immunosuppressed individuals. Infections with the potentially zoonotic C. muris and C. parvum have been reported in dogs and cats, but only rarely.
Taxonomy
Phylum: Alveolata
Sub-phylum: Apicomplexa
Class: Coccidea
Order: Cryptosporida
Although Cryptosporidium shares many characteristics with other gastrointestinal coccidia (e.g. Eimeria and Isospora), it has unique characteristics that set it apart from other coccidians, and shares some similarities with the gregarines, which are typically parasites of invertebrates.
On the basis of morphological, biological and molecular data, many species of Cryptosporidium are recognized in mammals, some of which are host-specific, as well as distinct species in birds, amphibians and reptiles, and fish. Additional genotypes and species are increasingly recognized with the application of molecular methods. For veterinarians a key issue is host specificity, particularly the ability of some species/genotypes of of Cryptosporidium to transmit between animals and people.
Surveys for Cryptosporidium in various parts of the world have shown C.canis to be the predominant species in dogs, and C. felis in cats. Cryptosporidium muris and C. parvum have also been recovered from dogs
Morphology
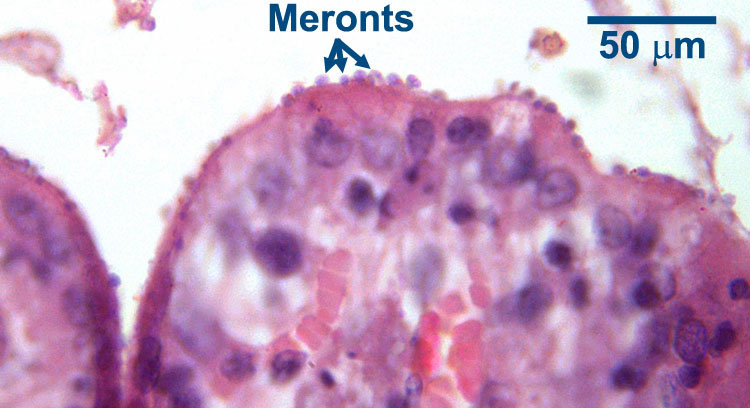
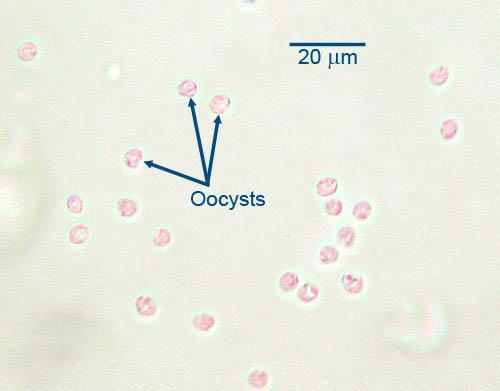
In mammalian hosts, intestinal Cryptosporidium develops within the microvilli on the luminal surface of the enterocytes. The presence of the parasites causes the microvilli to become distended, and once the oocyst is formed the microvillus is disrupted and the oocyst enters the intestinal lumen. With a light microscope it is very difficult or impossible to distinguish the various developmental stages of the parasite, but the distended microvilli signal its presence.
Host range and geographic distribution
In studies where species have been identified , the predominant species in cats is C. felis, and in dogs is C. canis. Very rarely, natural infections of dogs with C. muris or C. parvum have been reported. A study of young dogs (<1 year old) on Prince Edward Island found Cryptosporidium in 8% of 62 dogs from an animal shelter, and in 10% of 78 dogs from veterinary clinics. Isolates from the four animals were all C. canis.
Life cycle — direct
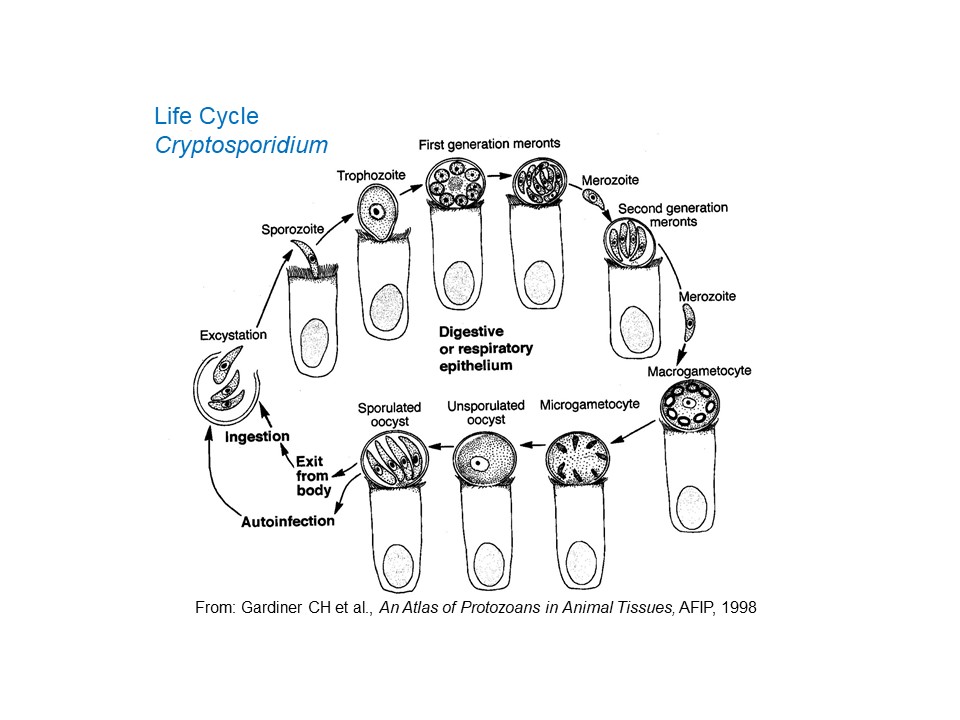
Following ingestion by a suitable host, the oocysts excyst and the sporozoites penetrate epithelial cells, in which they locate in the microvilli (where these are present) in parasitophorous vacuoles between the cell wall and the cytoplasm. They are, therefore, intracellular but extracytoplasmic. It is in this location that parasite development continues. First the sporozoites (at this stage sometimes called trophozoites) undergo a cycle of asexual reproduction to form merozoites which are contained within (Type I) meronts. The merozoites are then released, invade additional mucosal cells and undergo a second cycle of asexual reproduction to form either a second generation of Type I meronts, which can continue asexual reproduction, or Type II meronts. The merozoites from Type II meronts, when released, invade yet more cells and form the sexual stages of the parasite: macrogametes and microgametes. Fertilization of the macrogametes by the microgamoetes results in the production of oocysts which sporulate within the host and, as indicated above, are immediately infective. Typically, most of the oocysts produced are thick-walled and can survive very well in the external environment, but some are thin-walled and relatively fragile and have very poor survival outside the host. Usually these thin-walled oocysts release their sporozoites in the gut lumen of the host and these can result in (internal) autoinfection, potentially a major clinical problem especially in hosts that are immunosuppressed. Depending on host and parasite species, the pre-patent period of Cryptosporidium varies between two and 14 days.
Recent reports indicate that some species of Cryptosporidium (C. parvum from cattle and C. hominis) can complete its entire life cycle - oocyst to oocyst - in cell-free culture medium. The significance of this perhaps surprising finding, especially for the epidemiology of the parasite, has yet to be fully explored.
Epidemiology
Pathology and clinical signs
In dogs, infections with Cryptosporidium (most often C. canis) are sometimes associated with diarrhea, but many infected dogs remain asymptomatic even though they might continue to shed oocysts for months. The situation for cats (most often C. felis) is similar. For both dogs and cats, young animals are more likely to show clinical signs than are adults, but in cats intermittent clinical signs can continue for long periods. In immunocompetent hosts, clinical Cryptosporidium infection is usually self-limiting.
Diagnosis
Treatment and control
Control of the parasite depends primarily on maintaining a clean environment, particularly for young animals, Oocysts are immediately infective and can survive months to years in wet environments, including chlorinated water. Oocysts are killed by desiccation, temperatures below -20C, and above 65C. Disinfection requires cresols, phenols, or strong bleach concentrations once organic matter has been removed.
Public health significance
References
Uehlinger FD et al. (2013) Zoonotic potential of Giardia duodenalis and Cryptosporidium spp. and prevalence of intestinal parasites in young dogs from different populations on Prince Edward Island, Canada. Veterinary Parasitology (In Press)
Elwin K et al. (2012) The epidemiology of sporadic human infections with unusual cryptosporidia detected during routine typing in England and Wales, 2000-2008. Epidemiology and Infection 140: 673-683.Lucio-Forster A et al. (2010) Minimal zoonotic risk of cryptosporidiosis from pet dogs and cats. Trends in Parasitology 26: 174-179.
Fayer R, (2010) Taxonomy and delimitation in Cryptosporidium. Experimental Parasitology 124: 90-97.
Bowman DD et al. (2010) Cryptosporidiosis and giardiasis in dogs and cats: Veterinary and public health importance. Experimental Parasitology 124: 121-127.
Shukla R et al. (2006) Cryptosporidium spp. and other zoonotic enteric parasites in a sample of domestic dogs and cats in the Niagara region of Ontario.
Canadian Veterinary Journal 47: 1179-1184.
Barta JR et al. (2006) What is Cryptosporidium? Reappraising its biology and phylogenetic affinities. Trends in Parasitology 22: 463-468.

